Background: Diffuse large B-cell lymphoma (DLBCL) represents the most common subtype of non-Hodgkin lymphoma (NHL), comprising approximately 30-40% of new NHL cases. Although immunochemotherapy is the current front-line treatment for DLBCL, nearly 40% of patients will face disease relapse or refractoriness to the initial therapy, with a suboptimal prognosis. Gemcitabine in combination with oxaliplatin (GEMOX) offers an established treatment regimen with a manageable side-effect profile, thus providing a viable backbone for innovative treatment combinations. Linperlisib, a selective PI3Kδ inhibitor, has demonstrated its ability to inhibit the growth of PI3Kδ-expressing human tumor cells, as evidenced by both in vitro and in vivo studies. The tolerability, pharmacokinetics, and preliminary efficacy of linperlisib were affirmed in a preceding phase I study, laying the groundwork for its further exploration in patients with relapsed or refractory (r/r) DLBCL. This study aimed to investigate the efficacy and safety of combining linperlisib with GEMOX in treating r/r DLBCL patients.
Methods: In this multicenter, single-arm phase Ib/II trial (NCT04500561), histologically confirmed r/r DLBCL patients across six Chinese centers were enrolled and received oral linperlisib (80 mg/day) alongside intravenous GEMOX (gemcitabine 1000 mg/m 2 on day 1, and oxaliplatin 100 mg/m 2 on day 2) every three weeks, with GEMOX administered for a maximum of six cycles. The primary endpoint was the objective response rate (ORR), with secondary outcomes including disease control rate (DCR), time to response (TTR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS).
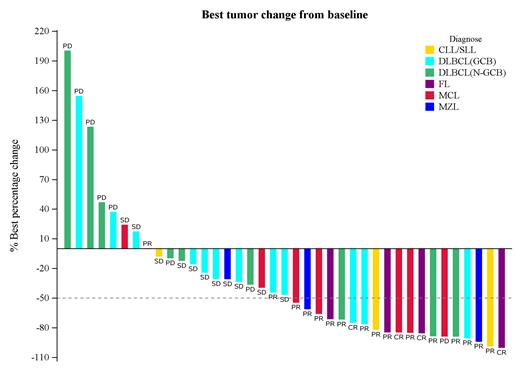
Results: Between September 2020 and June 2021, 39 patients were enrolled and received the study treatment, all of whom were included in the efficacy and safety analysis. By the data cutoff in February 2023, and after a median follow-up of 24.1 months, three patients were still on treatment. The ORR reached 53.8% (95%CI: 37.2, 69.9), and the DCR was recorded at 64.1% (95%CI: 47.2, 78.8) (Figure 1). The median TTR was 1.3 months (range: 1.2-4.0). The median DOR stood at 5.7 months (95%CI: 4.3-9.1), and the median PFS was 5.4 months (95%CI: 1.8-6.7). The median OS had not yet been reached by the cutoff, with the one-year OS rate standing at 65.5% (95%CI: 48.1-78.3). Stratification by subtype revealed an ORR of 66.7% (6/9) among germinal center B-cell like (GCB) DLBCL patients compared to 40% (10/25) among non-GCB DLBCL patients. Median PFS was reported at 3.0 and 3.4 months for GCB and non-GCB DLBCL patients, respectively. The most prevalent grade 3 or higher treatment-related adverse events included decreases in neutrophil count (46.2%), platelet count (41.0%), and white blood cell count (28.2%).
Conclusion: The combination of linperlisib with GEMOX exhibits a favorable safety profile and promising clinical efficacy in patients with r/r DLBCL who have received a median of two prior systemic therapies. These findings support the potential role of linperlisib combined with GEMOX as an active and safe therapeutic option for patients with r/r DLBCL, and set a foundation for further research into linperlisib's potential in combination with other treatment regimens.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Linperlisib, a selective PI3K inhibitor, has demonstrated its ability to inhibit the growth of PI3K-expressing human tumor cells, as evidenced by both in vitro and in vivo studies. The tolerability, pharmacokinetics, and preliminary efficacy of linperlisib were affirmed in a preceding phase I study in patients with relapsed or refractory B cell malignancies

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal